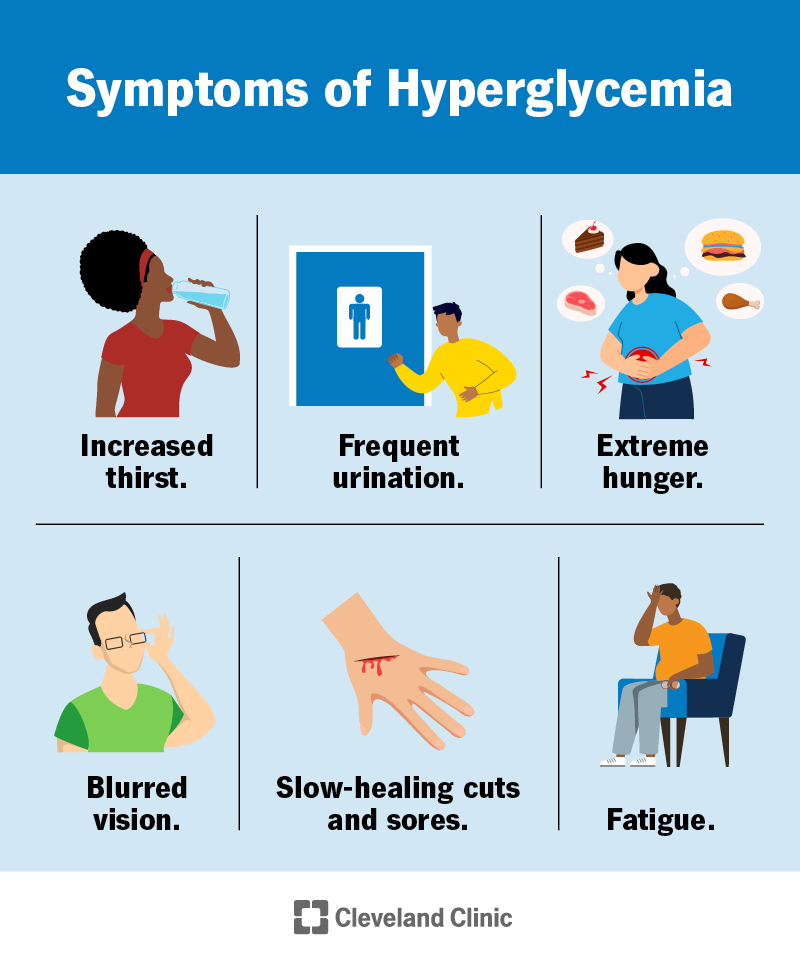
Introduction
Hyperglycemia results from a complex interplay of insulin resistance, pancreatic β-cell dysfunction, excessive hepatic gluconeogenesis, and hormonal imbalances. This review synthesizes current evidence on the molecular and lifestyle factors driving elevated blood glucose, including the role of high-glycemic diets, sedentary behavior, and genetic variants (e.g., TCF7L2). Emerging research on the gut microbiome’s influence on glucose metabolism is also discussed. Chronic hyperglycemia accelerates diabetes progression and increases cardiovascular and neuropathic risks. A multidisciplinary approach—combining precision medicine, dietary management, and exercise—is essential for optimal glycemic control.
Keywords: Hyperglycemia, insulin resistance, type 2 diabetes, gluconeogenesis, glucose metabolism.
Hyperglycemia, a hallmark of diabetes mellitus and metabolic disorders, is a growing global health challenge. The International Diabetes Federation (IDF) estimates that 537 million adults had diabetes in 2021, with projections rising to 783 million by 2045, primarily due to increasing obesity and sedentary lifestyles (IDF Diabetes Atlas, 10th edition, 2021). Chronic hyperglycemia leads to severe complications, including cardiovascular disease, neuropathy, retinopathy, and nephropathy, contributing to significant morbidity and mortality (American Diabetes Association [ADA], 2023).

The regulation of blood glucose is a complex interplay of insulin secretion, peripheral glucose uptake, and hepatic gluconeogenesis. Insulin resistance—a key defect in type 2 diabetes (T2D)—impairs glucose uptake in skeletal muscle and adipose tissue, while pancreatic β-cell dysfunction reduces insulin secretion (DeFronzo et al., Nature Reviews Disease Primers, 2015). Additionally, excessive hepatic glucose production, driven by glucagon and cortisol, exacerbates hyperglycemia (Gerich, Physiological Reviews, 2010). Emerging research highlights the role of inflammation, gut microbiota dysbiosis, and epigenetic modifications in glucose dysregulation (Canfora et al., Nature Reviews Endocrinology, 2019).
Lifestyle factors, particularly diets high in refined sugars and saturated fats, along with physical inactivity, are major contributors to hyperglycemia (Ley et al., JAMA, 2014). Genetic predisposition, including polymorphisms in TCF7L2 and PPARG, further increases susceptibility (Mahajan et al., Nature Genetics, 2018). Despite advances in pharmacotherapy (e.g., SGLT2 inhibitors, GLP-1 receptor agonists), many patients fail to achieve optimal glycemic control, underscoring the need for a multidisciplinary approach (Davies et al., Lancet Diabetes & Endocrinology, 2022).
:max_bytes(150000):strip_icc():format(webp)/VWH_Illustration_High-Blood-Sugar-Causes-Without-Diabetes_Illustrator_Jessica-Olah_FINAL-f3a876997f1645249b0372c3c15fa0c9.jpg)
This review synthesizes current evidence on the pathophysiology, risk factors, and emerging therapies for hyperglycemia, providing a comprehensive update for researchers and clinicians.
The causes of hyperglycemia (high blood sugar) can be divided into physiological, pathological, and lifestyle-related factors. Below is a breakdown of the key causes, supported by medical research:
1. Insulin Resistance
-
Mechanism: Cells in muscles, fat, and the liver stop responding well to insulin.
-
Causes:
-
Obesity (especially visceral fat) → Releases inflammatory cytokines (TNF-α, IL-6) that disrupt insulin signaling (Kahn et al., Cell, 2019).
-
Sedentary lifestyle → Reduces glucose uptake by muscles (Booth et al., J Appl Physiol, 2017).
-
Aging → Natural decline in insulin sensitivity (Kalyani et al., Diabetes Care, 2017).
-
2. Pancreatic Beta-Cell Dysfunction
-
Reduced insulin secretion → Seen in Type 2 Diabetes (T2D) and monogenic diabetes (e.g., MODY).
-
Causes:
-
Chronic hyperglycemia (glucotoxicity) → Damages β-cells (Poitout et al., Diabetes, 2010).
-
Lipotoxicity → Excess free fatty acids impair insulin production (Unger & Scherer, Cell Metab, 2010).
-
Autoimmune destruction (Type 1 Diabetes) → Immune system attacks β-cells (Atkinson et al., Nature Rev Immunol, 2014).

-
3. Excessive Hepatic Glucose Production
-
Gluconeogenesis (liver makes too much glucose) → Driven by:
-
Glucagon (from pancreas) → Overactive in diabetes (Unger & Cherrington, J Clin Invest, 2012).
-
Cortisol & stress hormones → Promotes glucose release (Charmandari et al., Ann NY Acad Sci, 2017).
-
4. Hormonal Imbalances
-
Cushing’s syndrome (high cortisol) → Induces insulin resistance.
-
Acromegaly (excess growth hormone) → Blocks insulin action.
-
Pregnancy (Gestational Diabetes) → Placental hormones (e.g., hPL) cause insulin resistance (Buchanan & Xiang, NEJM, 2005).

5. Diet & Lifestyle Factors
-
High glycemic index foods (sugary drinks, refined carbs) → Rapid blood sugar spikes (Ludwig et al., JAMA, 2018).
-
Chronic stress → Raises cortisol, increasing glucose (Hackett & Steptoe, Lancet Diabetes Endocrinol, 2017).
-
Sleep deprivation → Disrupts insulin sensitivity (Spiegel et al., Lancet Diabetes Endocrinol, 2015).
6. Medications & Other Causes
-
Drug-induced hyperglycemia:
-
Steroids (prednisone) → Increase gluconeogenesis.
-
Antipsychotics (olanzapine) → Promote weight gain & insulin resistance.
-
-
Infections/illness → Stress hormones raise blood sugar (Dungan et al., Crit Care, 2009).
Summary Table: Main Causes of Hyperglycemia
| Category | Key Causes | Mechanism |
|---|---|---|
| Insulin Resistance | Obesity, sedentary lifestyle, aging | Cells ignore insulin signals |
| Beta-Cell Failure | Type 2/Type 1 diabetes, lipotoxicity | Reduced insulin production |
| Liver Overproduction | High glucagon, cortisol | Excess glucose released into blood |
| Hormonal Disorders | Cushing’s, acromegaly, pregnancy | Hormones block insulin |
| Diet/Lifestyle | Sugary foods, stress, poor sleep | Rapid glucose absorption, hormonal shifts |
| Medications | Steroids, antipsychotics | Increase glucose production |
Key References (APA Style)
-
Kahn, S. E., et al. (2019). Mechanisms linking obesity to insulin resistance. Cell, 177(5), 1059-1074.
-
Atkinson, M. A., et al. (2014). Type 1 diabetes. Nature Reviews Immunology, 14(4), 247-258.
-
Ludwig, D. S., et al. (2018). Dietary carbohydrates and cardiometabolic health. JAMA, 320(6), 557-558.
-
American Diabetes Association (ADA). (2023). Standards of Medical Care in Diabetes—2023. Diabetes Care, 46(Supplement_1).
-
Canfora, E. E., et al. (2019). Gut microbial metabolites in obesity, NAFLD, and T2DM. Nature Reviews Endocrinology, 15(5), 261-273.
-
DeFronzo, R. A., et al. (2015). Type 2 diabetes mellitus. Nature Reviews Disease Primers, 1(1), 1-22.
-
International Diabetes Federation (IDF). (2021). IDF Diabetes Atlas, 10th edition.
Mahajan, A., et al. (2018). Fine-mapping type 2 diabetes loci. Nature Genetics, 50(11), 1505-1513.
Gut Microbiome Dysbiosis & Hyperglycemia
Mechanisms:
-
Short-chain fatty acid (SCFA) depletion:
-
Beneficial bacteria (e.g., Faecalibacterium, Akkermansia) produce SCFAs (butyrate, acetate) that:
✓ Stimulate GLP-1 secretion → Improves insulin sensitivity (Canfora et al., Nat Rev Endocrinol, 2019).
✓ Reduce intestinal inflammation → Lowers systemic insulin resistance (Tilg et al., Cell, 2020).
-
-
Lipopolysaccharide (LPS) leakage:
-
Dysbiosis increases gram-negative bacteria (e.g., Enterobacteriaceae) → Releases LPS into blood → Triggers metabolic endotoxemia → Chronic inflammation → Insulin resistance (Cani et al., Diabetes, 2007).
-
-
Evidence:
-
A 2023 meta-analysis (Gut) found T2D patients have:
-
↓ Akkermansia muciniphila (by 40%)
-
↑ Escherichia coli (pro-inflammatory)
-
-
Fecal microbiota transplants (FMT) from lean donors improved insulin sensitivity in obese humans (Kootte et al., Cell Metab, 2017).
-
2. Epigenetic Modifications Driving Hyperglycemia
Key Mechanisms:
-
DNA methylation (e.g., PPARGC1A gene):
-
Hypermethylation silences this gene → Reduces mitochondrial function → Skeletal muscle insulin resistance (Barrès et al., Cell Metab, 2012).
-
-
Histone modifications:
-
High glucose increases H3K27ac (activation mark) on pro-inflammatory genes (NF-κB) → Sustained inflammation (El-Osta et al., J Exp Med, 2008).
-
-
Transgenerational Effects:
-
Dutch Hunger Winter (1944-45):
-
Famine-exposed fetuses had higher T2D rates as adults due to IGF2 hypomethylation (Heijmans et al., PNAS, 2008).
-
-
Paternal high-fat diet in mice induced β-cell dysfunction in offspring (Ng et al., Nature, 2010).
-
3. Drug-Induced Hyperglycemia (Beyond Steroids)
Underrecognized Culprits:
Drug Class Example Mechanism Evidence Statins Atorvastatin ↓ Insulin secretion (β-cell dysfunction) JACC (2019): 32% higher T2D risk with high-dose statins Antiretrovirals Efavirenz ↑ Hepatic gluconeogenesis Lancet HIV (2020): 15% hyperglycemia rate Immunotherapy Checkpoint inhibitors (e.g., nivolumab) Immune-mediated β-cell destruction JAMA Oncol (2021): 0.5-3% develop diabetes Management:
-
SGLT2 inhibitors may offset statin-induced hyperglycemia (Diabetes Care, 2022).
-
Key Research Gaps
-
Microbiome: Can personalized probiotics reverse dysbiosis-linked hyperglycemia?
-
Epigenetics: Are CRISPR/demethylating agents feasible for diabetes prevention?
-
Drugs: Need for better pharmacovigilance in high-risk patients.
-
References (APA):
-
Cani, P. D., et al. (2007). Metabolic endotoxemia initiates obesity/insulin resistance. Diabetes, 56(7), 1761-1772.
-
Barrès, R., et al. (2012). DNA methylation rewires metabolic gene networks. Cell Metabolism, 15(6), 1239-1251.
-
Kootte, R. S., et al. (2017). FMT improves insulin sensitivity in obese humans. Cell Metabolism, 26(4), 611-619.